Abstract
Introduction: Anaplastic Large Cell Lymphoma, Anaplastic Lymphoma Kinase-negative (ALCL, ALK-) is a clinically aggressive lymphoid neoplasm with characteristic histologic and genetic features. The analysis from a retrospective International T-cell Lymphoma Project reported intermediate outcomes for ALCL, ALK- patients with 5-year event-free survival (EFS) and overall survival (OS) rates of 39% and 46%, respectively ( Savage et al. Blood 2008 ). In this analysis, we sought to study clinical features and outcomes of 203 patients prospectively enrolled into the international T-cell lymphoma registry, the T-cell Project (TCP).
Methods: The T-Cell Project prospectively registered consecutive patients with newly diagnosed peripheral T-cell lymphomas (PTCL) from 74 centres, in 14 countries, across 4 continents from Sep 2006 -Dec 2015. Demographic, clinical, and pathologic characteristics at the time of diagnosis, as well as treatment details and survival outcomes were collected. A key aim of this global collaborative project was to more precisely define clinical characteristics and outcome of patients with various subtypes of PTCL.
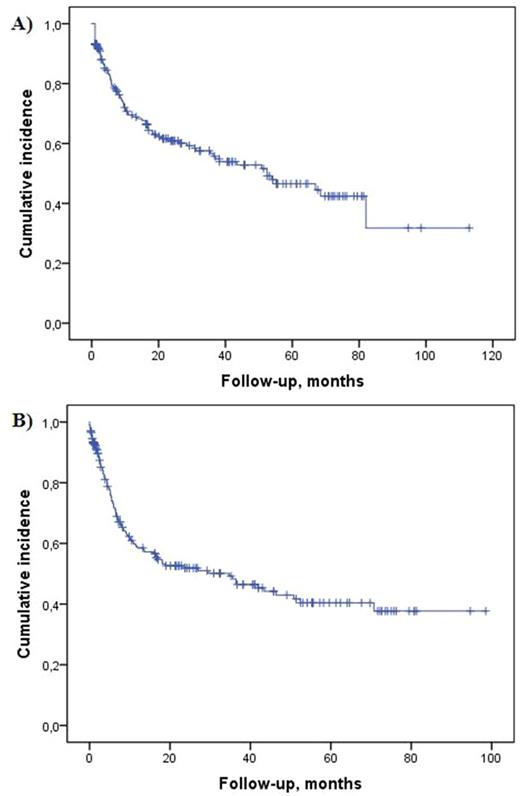
Results: From a total of 1,429 evaluable PTCL cases, 203 (14.8%) were confirmed as ALCL, ALK- following international histopathologic panel review. As anticipated, these cases, as a proportion of all PTCL cases, varied across geographical regions (Asia 4.6%, South America 29.3%, U.S.A 12.7%, and Europe 14.7%). The median age at diagnosis was 55 (range, 18-89) years with a slight male predominance (63%). Stage III/IV disease was identified in 61% of patients, whilst bulky disease >5cm (25%) and BM involvement (9%) were uncommon. 65% of patients presented with low IPI score. Data on therapy was available in 155 (76%) patients, of whom 142 (92%) received chemotherapy (CHT) as part of first-line treatment. 23 (14.8%) patients additionally received concurrent or sequential radiotherapy (RT), whilst 12 patients (7.7%) underwent high-dose therapy as consolidation. Eleven (7%) patients received palliative care only. Of 142 patients treated with chemotherapy, 137 (96.5%) received anthracycline-containing regimens. There was no significant variation in anthracycline use by global geographic regions. The investigator-assessed overall response rate was 72%, with 84 (58%) patients achieving complete remission. With a median follow-up of 41 (range, 31-51) months, the median PFS (n=114) was 35 (95% CI 16-53) months, and 3-year and 5-year PFS rates were 48% and 40%, respectively. The median OS (n=203) was 52 (95% CI, 29-76) months, and 3-year and 5-year OS rates were 57% and 47%, respectively (Fig.1). At the time of data cut off, 84 (41%) patients died. Cause of death was most commonly attributable to lymphoma (72%) and infection (8%).
Conclusions: This is the largest-to-date prospective international registry study of ALCL, ALK- patients describing epidemiology, clinical features, treatment approaches, and outcomes. With a mature follow-up, we report comparable results to previously reported from the retrospective International T-Cell Lymphoma Project. The long term survival rates after modern treatment approaches are disappointing with more than half of the patients not surviving past 5 years from diagnosis. This study underscores the urgent need for improved frontline therapies for ALCL, ALK- patients, and provides a robust platform for designing and interpreting future clinical trials. Recent results of incorporating novel CD30-targeting agent into initial therapy protocols are encouraging, and confirmatory randomized trial analysis is underway.
Figure 1. Kaplan Meier estimates of overall (A) and progression-free (B) survival among ALCL, ALK- patients enrolled into the prospective TCP registry.
Shustov: Celgene: Other: Publication assistance; Spectrum Pharmaceuticals: Consultancy, Research Funding. Horwitz: ADCT Therapeutics: Research Funding; Mundipharma: Consultancy; Kyowa-Hakka-Kirin: Consultancy, Research Funding; HUYA: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Aileron Therapeutics: Research Funding; BMS: Consultancy. Radford: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Consultancy; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding; Novartis: Speakers Bureau; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Speakers Bureau; Seattle Genetics: Speakers Bureau; GSK: Equity Ownership; AstraZeneca: Equity Ownership. Chiappella: Nanostring: Speakers Bureau; Amgen: Speakers Bureau; Pfizer: Speakers Bureau; Celgene: Speakers Bureau; Roche: Speakers Bureau; Teva: Speakers Bureau; Janssen: Speakers Bureau. Federico: takeda: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal